In the latest development in Alzheimer’s research, TIM-3 therapy for Alzheimer’s is emerging as a promising innovative approach to combat this devastating disease. Researchers have discovered that manipulating the TIM-3 checkpoint molecule can rejuvenate the brain’s immune response, specifically enabling microglia to efficiently attack and clear amyloid plaques that are characteristic of Alzheimer’s. This breakthrough highlights a significant shift in how we visualize Alzheimer’s treatment, transitioning from traditional methods to more immune-centric therapies. Understanding the interplay between TIM-3, microglia, and neuroinflammation therapy offers new pathways for improving cognitive function in affected individuals. By leveraging knowledge gained from cancer treatment strategies, TIM-3 therapy for Alzheimer’s aims not only to halt progression but to potentially restore memory and cognitive abilities.
Introducing TIM-3 immunotherapy for Alzheimer’s, researchers are exploring a pathway that leverages immune system responses similar to those used in cancer treatments. This approach focuses on the manipulation of checkpoint proteins, specifically TIM-3, to activate microglial cells, which are crucial for clearing brain plaques. Recent studies have aimed at discerning how these immune cells interact with neuroinflammation, offering fresh insights into reversing cognitive decline. By targeting molecules that inhibit immune activity, scientists believe they can overcome the barriers that prevent effective plaque clearance in Alzheimer’s patients. This emerging strategy could redefine therapeutic paradigms in Alzheimer’s, integrating cancer treatment methodologies to stimulate the brain’s natural defenses.
Understanding TIM-3 Therapy for Alzheimer’s Disease
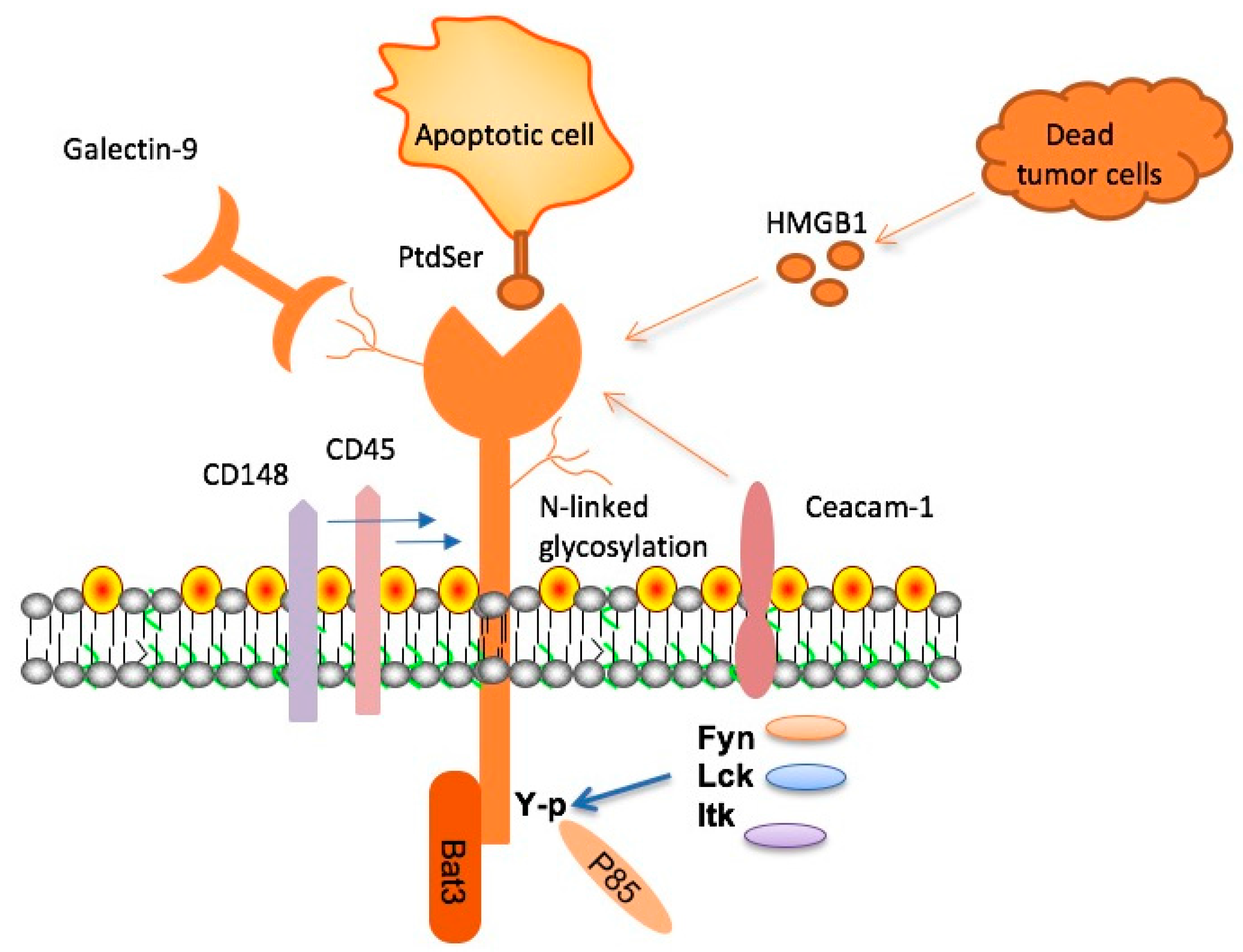
TIM-3 therapy for Alzheimer’s disease represents a groundbreaking advancement in Alzheimer’s research. By targeting the TIM-3 molecule, researchers are seeking to enhance the immune response of microglia—glial cells in the brain responsible for clearing amyloid plaques associated with Alzheimer’s. The cellular basis of Alzheimer’s involves the accumulation of these plaques which can hinder cognitive function. By inhibiting TIM-3, this therapy aims to release the inhibitory signals that prevent microglia from effectively engaging and eliminating these harmful plaques.
In studies involving mouse models, the deletion of the TIM-3 gene led to a significant improvement in plaque clearance and cognitive functions. In fact, the mice demonstrated better memory retention and navigation abilities after TIM-3 manipulation. This approach, which is being compared to cancer treatments utilizing checkpoint inhibitors, provides hope for novel therapeutic pathways in Alzheimer’s, especially for patients with late-onset Alzheimer’s disease, which constitutes over 90% of cases.
The Role of Immune System in Alzheimer’s Research
The immune system plays a critical role in Alzheimer’s disease, particularly through microglia, the brain’s resident immune cells. In healthy scenarios, these cells efficiently clear debris and regulate inflammation; however, in Alzheimer’s pathology, their function becomes impaired, leading to neuroinflammation and plaque accumulation. Research indicates that checkpoint molecules, such as TIM-3, act to suppress the activity of microglia, inhibiting their ability to manage amyloid plaques effectively.
Recent studies indicate that enhancing immune response in the brain, through mechanisms like TIM-3 inhibition, holds significant therapeutic potential. By allowing microglia to operate to their full capacity, we may reduce the neuroinflammation seen in Alzheimer’s and improve cognitive outcomes for affected individuals. The exploration of immune system strategies traditionally used in cancer treatment sheds light on innovative pathways to tackle neurodegenerative diseases.
Microglia and Amyloid Plaques: A Closer Look
Microglia, often referred to as the brain’s immune defenders, have a dual role in maintaining brain health and responding to pathological conditions such as Alzheimer’s disease. In cases of plaque accumulation, their functionality is compromised due to heightened expression of checkpoint molecules like TIM-3. This results in a failure to clear amyloid beta plaques, contributing to disease progression and cognitive decline.
Research suggests that eliminating or inhibiting TIM-3 can help restore the microglia’s ability to phagocytose plaques in the brain. In experimental setups, the absence of TIM-3 has shown to not only promote plaque clearance but also alter plaque characteristics, thereby potentially reversing some cognitive deficits typically seen in Alzheimer’s. Understanding these interactions between microglia and plaque dynamics is crucial for developing effective neuroinflammation therapies.
Challenges in Alzheimer’s Drug Development
The journey towards effective Alzheimer’s therapies has been fraught with challenges, particularly in the context of drug development. Traditional medications have often targeted amyloid plaques directly, yet many trials have failed to demonstrate significant clinical benefits. The issue lies partly in the complexity of Alzheimer’s pathology and the nuanced role of the immune system. As highlighted by recent findings, the focus on immune modulation—specifically targeting checkpoint molecules like TIM-3—presents a novel approach, offering a new paradigm in drug development.
Emerging research shows promise in repurposing existing anti-TIM-3 antibodies that have been previously developed for cancer treatment. These antibodies could potentially be tailored to manage the neuroinflammatory components of Alzheimer’s. Such strategies aim to harness the body’s own immune response to combat the disease more effectively, signaling a shift toward innovative immune-centered treatment frameworks.
The Future of Alzheimer’s Treatment Strategies
Looking ahead, the integration of TIM-3 therapy and related immune strategies into Alzheimer’s treatment protocols reflects a shift in how we understand and approach neurodegenerative diseases. As researchers continue to investigate the mechanisms of TIM-3 and similar pathways, there is significant excitement around the potential for lasting cognitive improvements among Alzheimer’s patients. Current studies involving genetically engineered mouse models expressing human TIM-3 provide crucial insights that will inform future clinical trials.
Successful implementation of TIM-3 targeted therapies could also lead to a broader understanding of how neuroinflammation and synaptic health interplay in Alzheimer’s disease. With continued research, it is hoped that new therapeutic options will emerge, allowing for more effective management of Alzheimer’s and possibly slowing the disease’s progression, thus dramatically improving patient’s quality of life.
Neuroinflammation Therapy: Innovations in Alzheimer’s Care
Neuroinflammation therapy represents an innovative landscape in Alzheimer’s care, shifting the focus from just targeting amyloid plaques to addressing the underlying inflammatory processes that drive the pathology. The role of microglia, when they become activated, plays a significant part in this inflammation, thereby influencing disease progression. Enhancing our understanding of these mechanisms can potentially lead to more effective treatments that target inflammation alongside plaque management.
Recent studies have demonstrated how modulation of immune checkpoints like TIM-3 could prevent pathological inflammation and restore microglial function. Therapeutics aiming to reduce neuroinflammation may provide therapeutic benefits beyond mere plaque reduction, helping to preserve cognitive functions in Alzheimer’s patients. This multifaceted approach represents a newfound optimism in therapeutic strategies that address not only the symptoms but also the underlying immune dysregulation associated with Alzheimer’s.
Clinical Applications of Anti-TIM-3 Antibody
The potential clinical applications of anti-TIM-3 antibodies in Alzheimer’s disease treatment are garnering significant interest. These antibodies, previously designed to modify immune responses in cancer therapy, are now being evaluated for their efficacy in modulating the immune environment in the brains of Alzheimer’s patients. By blocking the negative regulatory effects of TIM-3 on microglia, these therapies could enable the brain’s immune cells to better manage plaque and prevent cognitive decline.
Clinical trials are expected to explore the safety and efficacy of these therapies, paving the way for new treatment paradigms. Moreover, the specific targeting of TIM-3 might reduce the risk of adverse effects, a common concern in Alzheimer’s drug development, where systemic immune suppression can lead to serious complications. Ensuring that anti-TIM-3 antibodies can effectively penetrate and act within the brain’s unique environment will be a crucial step in validating this therapeutic approach.
Emerging Trends in Alzheimer’s Research
As we delve deeper into Alzheimer’s research, emerging trends point towards a more nuanced understanding of neurodegeneration. The intersection of immune response dynamics, particularly through checkpoint inhibitors like TIM-3, highlights the complexity of Alzheimer’s pathology. This multi-dimensional approach is critical as researchers aim to identify biomarkers and novel therapeutic targets that can lead to earlier and more effective interventions.
Additionally, cross-disciplinary collaborations between neurobiology, immunology, and pharmacology are becoming increasingly evident. Such integrative research efforts are essential in developing comprehensive models of Alzheimer’s disease that consider both the neuroinflammatory aspects and the consequences of neurodegeneration. As this landscape continues to evolve, it holds the promise of transformative changes in how we approach diagnosis and treatment in Alzheimer’s disease.
Conclusion: The Promise of TIM-3 Insights in Alzheimer’s Therapy
In conclusion, insights gained from research on TIM-3 and its implications for Alzheimer’s therapy mark a significant step forward in the fight against this debilitating condition. By understanding the role of checkpoint molecules within the immune system, particularly in the context of microglial activity, we open new avenues for treatment strategies that harness the brain’s own defenses against amyloid plaque accumulation.
As studies progress and clinical trials begin, the hope is to establish TIM-3 therapy as a cornerstone in Alzheimer’s treatment protocols. This promising direction not only emphasizes innovative therapeutic targets, but also showcases the potential of rethinking how we engage the immune system in combating neurodegenerative diseases. The future of Alzheimer’s care may very well depend on this paradigm shift towards immune system modulation.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s and how does it work?
TIM-3 therapy for Alzheimer’s involves targeting the TIM-3 checkpoint molecule that inhibits microglia, the brain’s immune cells. By blocking TIM-3’s inhibitory function, these microglia can more effectively clear amyloid plaques associated with Alzheimer’s, potentially leading to cognitive improvements.
How does TIM-3 influence microglia in Alzheimer’s disease?
In Alzheimer’s disease, TIM-3 expression on microglia prevents them from engulfing harmful amyloid plaques. By inhibiting TIM-3, microglia can regain their ability to clear these plaques, which is crucial for alleviating neuroinflammation and restoring memory function.
What are the benefits of TIM-3 therapy compared to other Alzheimer’s treatments?
TIM-3 therapy has shown potential advantages over traditional Alzheimer’s treatments by selectively targeting the clearance of plaques without the adverse effects seen with some anti-amyloid antibodies, which can cause vascular damage. This focused strategy may improve cognitive outcomes with fewer risks.
Is TIM-3 therapy related to cancer treatment strategies?
Yes, TIM-3 therapy draws on immune system strategies developed in cancer treatment. Similar to how anti-TIM-3 antibodies are used to enhance immune responses against tumors, they may be repurposed to activate microglial clearance in Alzheimer’s, highlighting the overlap between cancer and Alzheimer’s research.
What role does neuroinflammation play in Alzheimer’s and how can TIM-3 therapy help?
Neuroinflammation is a significant factor in Alzheimer’s pathology, often exacerbated by plaque accumulation. TIM-3 therapy can help mitigate neuroinflammation by ensuring that microglia are active and clearing plaques rather than becoming dysfunctional, which could improve overall brain health.
How has recent Alzheimer’s research involving TIM-3 shown promise in animal models?
Recent studies demonstrated that deleting TIM-3 in mice led to improved plaque clearance and cognitive function. These findings suggest that TIM-3 therapy for Alzheimer’s could enhance memory and reduce symptoms by rejuvenating the brain’s immune response.
What future steps are being taken to develop TIM-3 therapy for human Alzheimer’s patients?
Researchers are currently testing human anti-TIM-3 antibodies in mouse models genetically modified to develop Alzheimer’s plaques. This step is crucial for assessing the therapy’s efficacy and safety before advancing into human clinical trials.
What percentage of Alzheimer’s cases are related to late-onset and TIM-3?
Approximately 90-95% of Alzheimer’s cases are classified as late-onset, making the TIM-3 gene, linked to this type through genome-wide studies, a critical focus for understanding the disease and developing targeted therapies.
Can TIM-3 therapy reverse memory loss in Alzheimer’s patients?
While TIM-3 therapy has shown promising results in mouse models by restoring some cognitive functions, it’s still being studied in humans. The goal is to enhance cognitive abilities by effectively targeting plaque-related issues.
What genetic factors are associated with TIM-3 in Alzheimer’s disease?
A specific polymorphism in the TIM-3 gene (HAVCR2) has been linked to an increased risk of late-onset Alzheimer’s. This genetic factor signifies the importance of TIM-3 in the disease’s pathology and could offer insights for future therapies.
| Key Point | Details |
|---|---|
| TIM-3 and Alzheimer’s Connection | Research shows TIM-3 is linked to late-onset Alzheimer’s disease and inhibits microglia from clearing amyloid plaques. |
| Microglia’s Role | Microglia are brain immune cells that help remove debris but are inhibited by TIM-3 from attacking plaques. |
| Research Methods | Scientists deleted TIM-3 gene in mouse models, leading to enhanced plaque clearance and improved cognitive function. |
| Potential Therapy | Future treatments might involve anti-TIM-3 antibodies to block its inhibitory effects on microglia in humans. |
| Recent Findings | The study implies that existing anti-TIM-3 therapies can be repurposed to target Alzheimer’s-related plaques. |
Summary
TIM-3 therapy for Alzheimer’s is an innovative approach that utilizes immune system strategies previously successful in cancer treatments. This groundbreaking research indicates that TIM-3 molecules, which act as checkpoint inhibitors, could be targeted to liberate microglia in the brain, allowing them to effectively clear harmful plaques associated with Alzheimer’s disease. By genetically modifying mice to lack TIM-3, researchers demonstrated improved cognitive abilities and significant plaque clearance. This discovery paves the way for developing specific therapies targeting TIM-3 in humans, potentially revitalizing treatment options for Alzheimer’s patients.