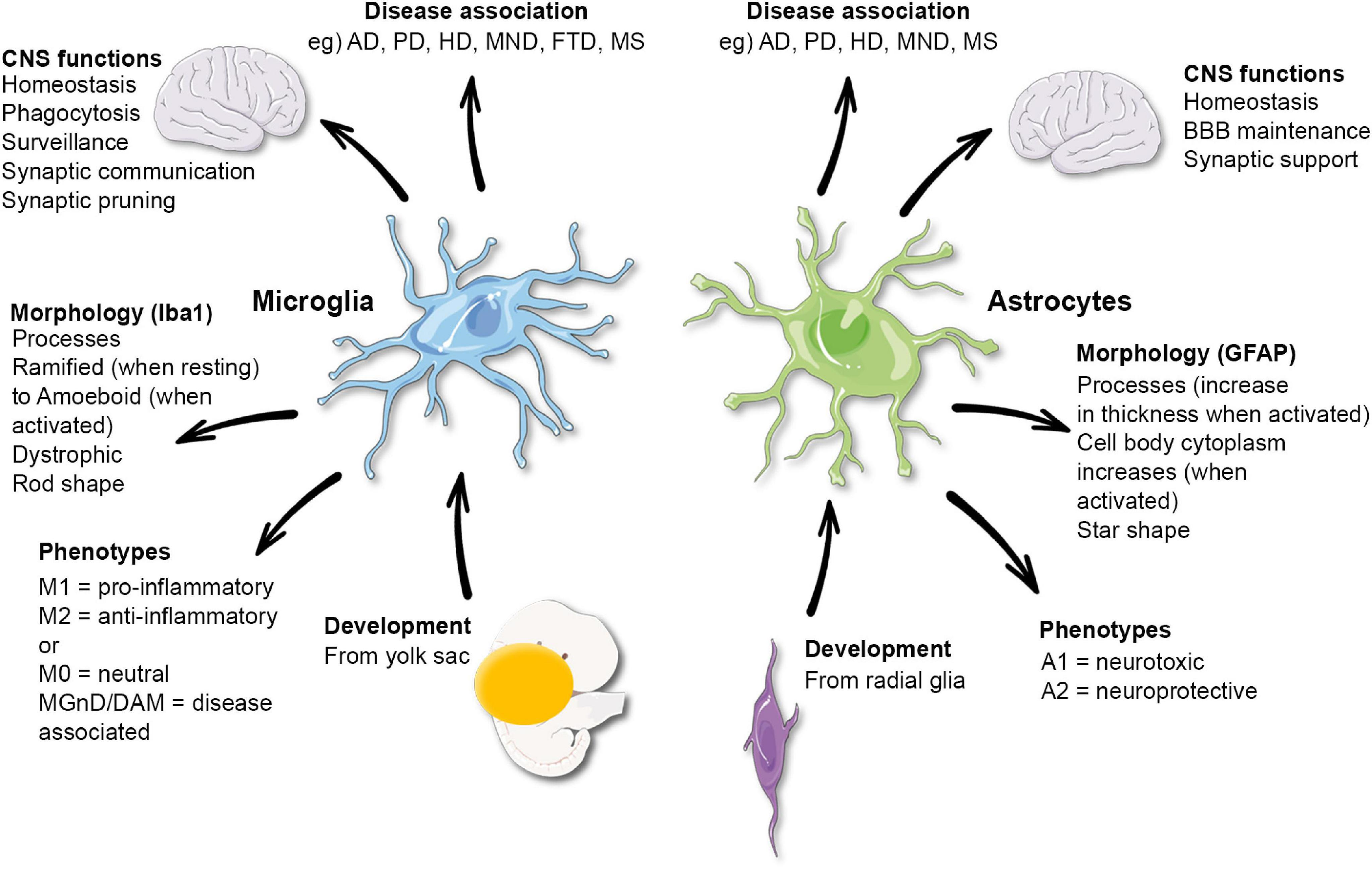
Microglial cells play a crucial role in maintaining brain health, acting as the primary immune defense within the central nervous system. These remarkable cells constantly survey their environment, ready to respond to injury or disease, and are fundamental in processes such as synaptic pruning. In the realm of Alzheimer’s research, Beth Stevens and her team have illuminated the dual nature of microglia, showing how their protective actions can sometimes inadvertently contribute to neurodegenerative diseases like Alzheimer’s and Huntington’s. By understanding the delicate balance microglial cells maintain during development and disease, researchers hope to develop targeted therapies that can mitigate their harmful effects while enhancing their beneficial functions. This transformative work not only deepens our understanding of the brain’s immune system but also opens new pathways for treating millions suffering from neurodegenerative disorders.
Glial cells, particularly microglia, are pivotal in neuroprotection and neural maintenance, acting as sentinels within the brain’s immune framework. These cells exhibit a fascinating ability to adapt their functions based on physiological demands, especially during synaptic remodeling which is essential for learning and memory. Recent studies spearheaded by neurobiologist Beth Stevens highlight how glial dysfunction can lead to adverse outcomes in conditions such as Alzheimer’s disease, emphasizing a significant focus on understanding brain immunity in health and disease. By uncovering the complexities of these immune cells, researchers are paving the way for innovative strategies that could transform treatment protocols for neurodegenerative conditions. This ongoing exploration into microglial dynamics not only sheds light on their role in cognitive health but could ultimately unlock potential therapies for the millions affected by such devastating diseases.
The Role of Microglial Cells in the Brain’s Immune System
Microglial cells are often described as the brain’s own immune system, tasked with the critical role of maintaining neural health. These cells continuously monitor the brain environment, searching for signs of injury or disease. When they identify broken or dying neurons, microglia spring into action to clear debris and perform a process known as synaptic pruning. This process involves the removal of weak or unnecessary synapses, ensuring that neural connections remain strong and efficient. However, if microglial activity becomes dysregulated, as seen in neurodegenerative diseases like Alzheimer’s, the balance of synaptic pruning can veer toward overactivity, leading to harmful consequences for brain function.
The research led by Beth Stevens has shed light on how microglial cells contribute not just to synaptic health but also to the progression of neurodegenerative disorders. When these cells inadvertently intensify their pruning processes, they can exacerbate conditions like Alzheimer’s disease and Huntington’s disease. Understanding this delicate balance of microglial activity is crucial, as it opens avenues for developing biomarkers that can diagnose these diseases earlier and more effectively. Through her pioneering research, Stevens demonstrates that investigating the minute aspects of brain biology, such as microglial function, can unearth significant insights into complex diseases.
Synaptic Pruning and Its Implication in Neurodegenerative Diseases
Synaptic pruning is a critical process in the development and functioning of the nervous system. It involves the elimination of unneeded synapses to streamline communication between neurons, which is essential for learning and memory. However, in the context of Alzheimer’s disease and other neurodegenerative disorders, this process can become dysregulated. Research has shown that overly aggressive synaptic pruning by hyperactive microglial cells may lead to the loss of key neural connections, which contributes to cognitive decline. This highlights the importance of maintaining a healthy balance of synaptic pruning as part of the brain’s immune response.
Beth Stevens’ work has been pioneering in uncovering the dysfunctional roles of microglial cells in enhancing the progression of neurodegenerative diseases. By studying how these cells interact with neurons throughout the pruning process, researchers are gaining a clearer picture of the underlying mechanisms that lead to conditions like Alzheimer’s. This has profound implications for future research directions, as targeting the molecular pathways involved in microglial pruning could potentially mitigate the effects of these devastating diseases. Thus, understanding the dual role of synaptic pruning—its necessity in brain health and its potential for harm in disease contexts—remains a major focus in Alzheimer’s research.
The Impact of Federal Funding on Alzheimer’s Research
Federal funding has been instrumental in advancing research in Alzheimer’s and other neurodegenerative diseases. Beth Stevens highlighted how her early research was almost entirely supported by grants from the National Institutes of Health, which allowed her to explore the role of microglial cells in synaptic pruning without the immediate pressure of commercial viability. This type of funding encourages scientists to delve deeper into basic science, fostering discoveries that might not have direct applications immediately but are critical in laying the groundwork for future advancements in therapies and understanding.
Furthermore, sustained federal support helps to create a collaborative environment where researchers can share findings and insights across disciplines. In the realm of Alzheimer’s research, this pooling of resources and knowledge enhances the potential to identify novel biomarkers and treatments. Stevens underscores that the collaborative nature of federally funded research not only accelerates discovery but also fosters a community of scientists dedicated to unraveling the complexities of neurodegenerative diseases in meaningful ways. As the population ages, continued investment in such research becomes even more essential.
The Need for Curiosity in Scientific Research and Discovery Paths for Alzheimer’s Treatment Opportunities and Challenges in the Study of Brain Diseases
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are crucial in Alzheimer’s research as they act as the brain’s immune system. They monitor brain health by removing dead or damaged cells and participating in synaptic pruning, which is essential for cognitive function. Abnormal microglial activity can contribute to the progression of Alzheimer’s and other neurodegenerative diseases, making them a key focus for scientists like Beth Stevens.
How do microglial cells contribute to neurodegenerative diseases?
Microglial cells contribute to neurodegenerative diseases by engaging in processes such as synaptic pruning and inflammation. In conditions like Alzheimer’s and Huntington’s diseases, these cells can mismanage the removal of synapses, leading to neuronal loss and impaired brain function. Understanding their roles helps researchers develop new therapeutic strategies.
Why are microglial cells considered part of the brain’s immune system?
Microglial cells are considered part of the brain’s immune system because they are the primary responders to injuries and infections in the central nervous system. They protect the brain by clearing debris, regulating inflammation, and maintaining homeostasis, which is vital for preventing neurodegenerative diseases like Alzheimer’s.
What is synaptic pruning and how do microglial cells affect it?
Synaptic pruning is the process of eliminating excess synapses to refine neural circuits, which is crucial for effective brain function. Microglial cells facilitate this process by selectively removing synapses that are weak or unnecessary. However, aberrant pruning by microglia could lead to neurodegenerative diseases such as Alzheimer’s, highlighting their dual role in brain health and disease.
Who is Beth Stevens and what is her contribution to the study of microglial cells?
Beth Stevens is a prominent neuroscientist recognized for her pioneering research on microglial cells and their implications in Alzheimer’s disease and other neurodegenerative disorders. Her work has reshaped our understanding of how these immune cells affect synaptic pruning and overall brain health, leading to potential new treatments and biomarkers.
What are the implications of studying microglial cells for Alzheimer’s treatment?
Studying microglial cells has significant implications for Alzheimer’s treatment, as it provides insights into the pathology of the disease. By understanding how microglia misfunction in Alzheimer’s, researchers like Beth Stevens aim to develop targeted therapies that can restore normal microglial activity, potentially slowing down or halting disease progression.
How do microglial cells relate to the concept of the brain’s immune system?
Microglial cells are integral to the brain’s immune system as they monitor and respond to changes in neural health. They are responsible for detecting and addressing issues like cellular debris or pathogens, thus playing a vital role in maintaining brain integrity and preventing neurodegenerative diseases like Alzheimer’s.
What recent advancements have been made in microglial research in relation to Alzheimer’s disease?
Recent advancements in microglial research, particularly by scientists like Beth Stevens, have revealed how these cells can contribute to the pathology of Alzheimer’s through aberrant synaptic pruning and inflammation. This research is paving the way for new biomarkers and potential therapies that could mitigate the effects of neurodegenerative diseases.
Can studying microglial cells lead to breakthroughs in neurodegenerative disease therapies?
Yes, studying microglial cells can lead to breakthroughs in neurodegenerative disease therapies. Understanding their role in synaptic pruning and immune responses provides a foundation for developing innovative treatments and biomarkers that could significantly impact the management of diseases like Alzheimer’s.
| Key Point | Details |
|---|---|
| Role of Microglial Cells | Act as the brain’s immune system, clearing out dead cells and helping in synaptic pruning. |
| Impact on Alzheimer’s Disease | Aberrant microglial pruning can contribute to Alzheimer’s and other neurodegenerative diseases. |
| Research Foundation | Beth Stevens’ research has laid a foundation for new biomarkers and treatments for Alzheimer’s. |
| Scientific Curiosity | Initial research was guided by curiosity and supported by federal funding, leading to significant discoveries. |
| Future Implications | Understanding microglial function enhances potential treatments for the 7 million Americans living with Alzheimer’s. |
Summary
Microglial cells play a crucial role in maintaining brain health as the brain’s immune system. Their function in clearing dead cells and pruning synapses is vital for neural communication. Recent research led by Beth Stevens has revealed that dysfunctional microglial activity may contribute to serious neurodegenerative diseases such as Alzheimer’s. This highlights the importance of continued investigation into microglial cells, as such studies pave the way for new treatment options and biomarkers that could significantly improve the lives of those affected by these conditions.