Alzheimer’s disease research is at the forefront of a critical battle against one of the most devastating neurodegenerative diseases affecting millions. Leading the charge is neuroscientist Beth Stevens, whose groundbreaking work on microglial cells has revealed their crucial role in brain health and disease. These immune cells, essential for clearing out dead neurons and maintaining synaptic function, often falter in conditions like Alzheimer’s, leading to its progression. With Stevens’ innovative strategies, new avenues for early Alzheimer detection and potential treatments are emerging, fueled by the latest neuroscience breakthroughs. As the aging population grows, this research could reshape the landscape of Alzheimer’s care, aiming to significantly reduce the escalating costs of this condition in the coming decades.
The exploration of Alzheimer’s disease, a complex condition characterized by cognitive decline and memory loss, is a vital area of scientific inquiry. Symbolizing hope in the face of neurodegenerative disorders, researchers like Beth Stevens are unraveling the functions of microglial cells, which serve as guardians of brain health. As they investigate the implications of abnormal cellular behavior, the quest for innovative therapies and methods for early detection becomes increasingly urgent. This relentless pursuit not only broadens our understanding of the disease but also opens doors to significant breakthroughs in treatment strategies. Ultimately, such endeavors manifest the critical intersection of basic science and practical application, aiming to alleviate the burdens of Alzheimer’s on individuals and society.
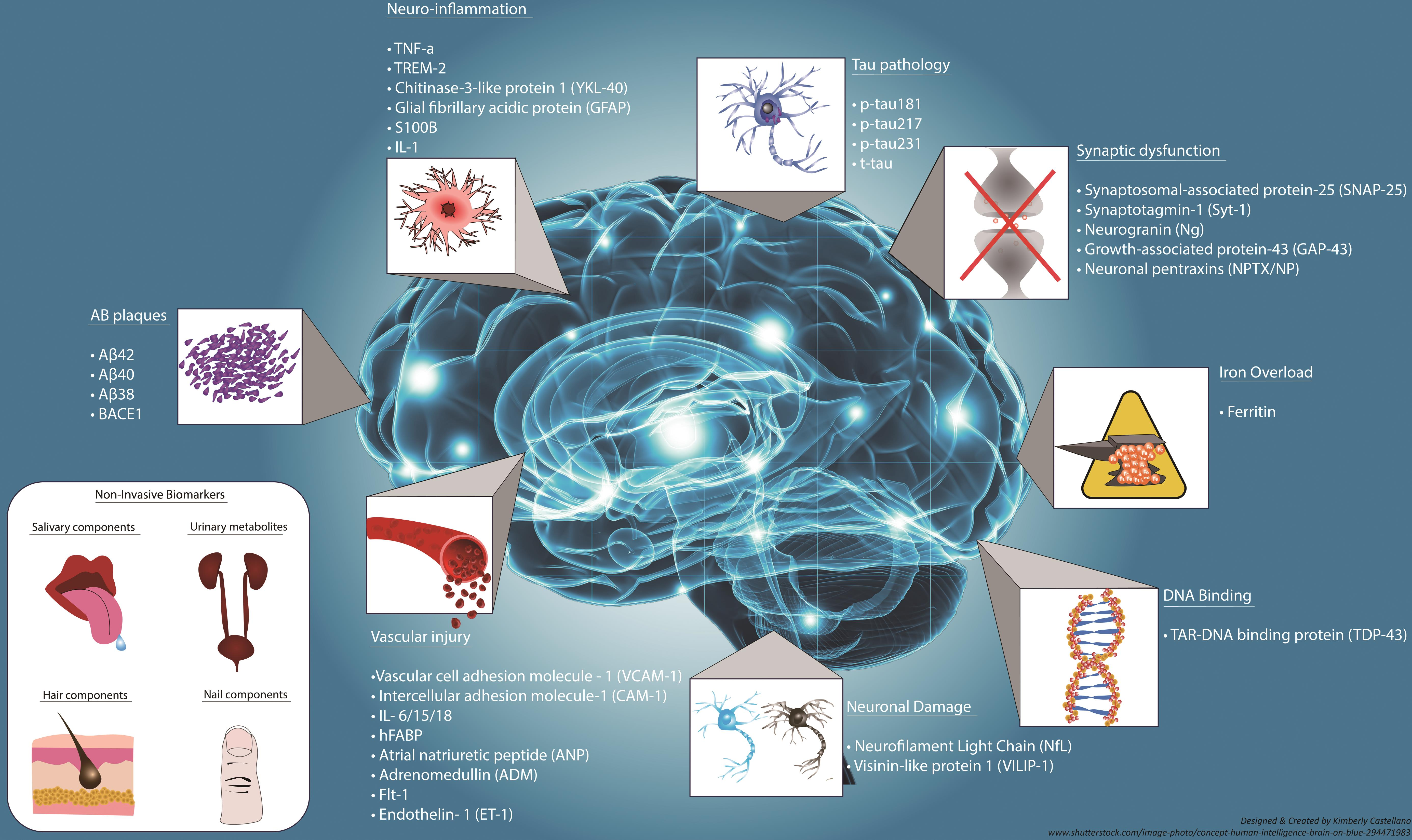
Understanding Microglial Cells and Their Role in Alzheimer’s Disease
Microglial cells play a crucial role in the brain’s immune defense system. These specialized cells continuously monitor the cerebral environment for signs of damage or disease, engaging in the cleanup of cellular debris. In the context of Alzheimer’s disease, research has revealed that microglial cells may become dysfunctional, leading to abnormal synaptic pruning. This flawed process can contribute to the neurodegenerative nature of Alzheimer’s, resulting in cognitive decline and increased neuronal damage. As such, advancements in our understanding of microglial behavior are critical for developing therapeutic strategies.
Beth Stevens’ groundbreaking research has shed light on how these cells interact with synapses and their influence on neurodevelopment. By decoding the mechanisms behind microglial activity, Stevens has linked its malfunctions to various neurodegenerative diseases, including Alzheimer’s. Modern neuroscience breakthroughs have opened up paths for novel treatments, emphasizing the importance of microglial cells in both disease progression and potential intervention.
Beth Stevens’ Impact on Alzheimer’s Disease Research
Beth Stevens has emerged as a leading figure in Alzheimer’s disease research, primarily due to her innovative approaches in studying microglial cells. Her lab at Boston Children’s Hospital has pioneered the exploration of how these immune cells regulate synaptic health and are implicated in neurodegenerative diseases. Stevens’ findings indicate that understanding these pathways can pave the way for the development of biomarkers for early Alzheimer detection. Early intervention in neurodegenerative diseases could drastically change the scope of treatment options available to patients.
The implications of Stevens’ work extend far beyond her laboratory. By targeting the early stages of neurodegeneration, her research aligns with growing endeavors in neuroscience to create proactive strategies in treating Alzheimer’s. With statistics predicting a significant rise in Alzheimer’s cases by 2050, Stevens’ investigation into microglial functioning is not just timely but essential in formulating effective public health responses.
The Future of Alzheimer’s Disease Treatment: Early Detection and Intervention
As research progresses, the focus on early Alzheimer detection has gained considerable momentum in the scientific community. Stevens’s work highlights that identifying abnormalities in microglial function could serve as a critical marker for early disease intervention. This proactive approach is crucial considering the anticipated increase in Alzheimer’s diagnoses. With almost 7 million Americans currently affected by Alzheimer’s, enhanced early detection methods could lead to timelier treatments that slow disease progression.
Moreover, leveraging findings from studies on microglial cells opens avenues for developing medications that aim to optimize brain health from its earliest stages. By combining efforts in research, like those found in Stevens’ lab, with the latest advancements in neuroscience, there is potential to mitigate the impact of Alzheimer’s disease significantly. Creating a preventive landscape against neurodegenerative diseases thus becomes an achievable goal.
Neuroscience Breakthroughs Revolutionizing Alzheimer’s Understanding
Recent neuroscience breakthroughs have changed the landscape of Alzheimer’s research significantly. The advent of advanced imaging techniques and genetic analyses has allowed scientists to observe the brain’s intricate workings more closely. These methods have revealed how microglial cells not only react to neurodegeneration but may also play a central role in how Alzheimer’s disease evolves. By aligning cutting-edge technology with biological insights, researchers like Beth Stevens are paving the way to decipher complex pathways that lead to effective treatments.
Furthermore, the collaboration between various research institutes and healthcare facilities has accelerated the pace of discovery, generating a wealth of knowledge about neurodegenerative diseases. The interconnectedness of their findings supports a holistic understanding of brain health and disease, illustrating that strategic alliances in neuroscience can amplify innovation and lead to impactful therapeutic solutions.
The Role of Federal Funding in Alzheimer’s Research
Federal funding has been instrumental in advancing Alzheimer’s disease research. As emphasized by Beth Stevens, support from government agencies enables researchers to explore fundamental questions that can lead to significant breakthroughs. In particular, the National Institutes of Health (NIH) plays a vital role in providing the necessary resources to investigate the complexities of neurodegenerative diseases, including Alzheimer’s.
Without this financial backing, many promising studies would face barriers that could slow down the efforts needed to combat diseases such as Alzheimer’s. Effective funding allows scientists to delve deeply into the workings of microglial cells and other mechanisms, fostering innovation that could potentially lead to more effective treatments and, eventually, a cure for Alzheimer’s.
Innovative Biomarkers for Early Alzheimer Detection
The development of innovative biomarkers stands as a cornerstone in the race against Alzheimer’s disease. Understanding the functioning of microglial cells can identify specific changes in brain activity that indicate the early onset of Alzheimer’s. By integrating findings from research studies, like those conducted by Beth Stevens, the potential for early diagnosis becomes increasingly realistic.
These biomarkers not only enhance the accuracy of early Alzheimer detection but could also guide the development of tailored therapeutics. By allowing healthcare professionals to intervene sooner, it might be possible to manage the symptoms more effectively and improve patients’ quality of life significantly.
Exploring the Interplay Between Genetics and Alzheimer’s
Genetics plays a critical role in understanding the risk factors associated with Alzheimer’s disease. Research has identified several genetic markers that can elevate the likelihood of developing this neurodegenerative condition. By exploring the interplay between genetics, microglial function, and disease pathology, researchers can develop comprehensive models that elucidate how these factors converge to influence Alzheimer’s disease progression.
The work of scientists like Beth Stevens illustrates the importance of integrating genetic research with neurological studies to innovate treatment and prevention strategies. This multi-faceted approach leads to a deeper understanding of how both intrinsic and extrinsic factors contribute to the disease landscape, offering hope for tailored therapies based on individual genetic predispositions.
The Neuroscience Community’s Response to Alzheimer’s Challenges
The neuroscience community has rallied in response to the growing challenges posed by Alzheimer’s disease, with initiatives focusing on collaboration and innovative research strategies. The recognition of microglial cells in disease mechanisms has led to more comprehensive studies, uniting scientists across various disciplines to solve this critical public health issue. Beth Stevens’ work exemplifies the collaborative spirit within neuroscience, showcasing how various research expertise can converge to tackle Alzheimer’s.
Additionally, the community’s commitment to understanding neurodegenerative diseases is reflected in the increased funding and resources being allocated towards such research endeavors. As collaborative efforts continue to gain momentum, there is a growing hope for advancements in treatment protocols that could ultimately lead to improved outcomes for those affected by Alzheimer’s.
Patient-Centric Approaches to Neurodegenerative Disease Care
Patient-centric approaches are becoming a foundational aspect of care in Alzheimer’s disease and other neurodegenerative conditions. Advocating for the voices of patients and their families in research initiatives is crucial, as it ensures that treatments align with their needs and experiences. By emphasizing patient perspectives, scientists can better tailor interventions that are not only scientifically sound but also emotionally supportive.
Research led by figures like Beth Stevens fosters this patient-centric paradigm by creating therapies that aim to enhance overall quality of life for those affected by Alzheimer’s. Understanding that every individual’s experience with the disease can vary significantly allows for a more nuanced approach to treatment and care.
Frequently Asked Questions
How is Beth Stevens’ research advancing Alzheimer’s disease research?
Beth Stevens’ research is pioneering in the field of Alzheimer’s disease research as it focuses on understanding microglial cells, the brain’s immune system. By revealing how abnormal pruning by microglia can contribute to neurodegenerative diseases like Alzheimer’s, her work lays the groundwork for new medications and early detection biomarkers.
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are vital in Alzheimer’s disease research because they act as the brain’s immune system, removing dead cells and trimming synapses. Stevens’ research indicates that dysfunctional pruning by microglia may drive the pathology of Alzheimer’s, making these cells a significant target for therapeutic interventions.
What are the implications of early Alzheimer detection in current research?
Early Alzheimer detection is crucial in Alzheimer’s disease research as it allows for timely interventions. Research led by scientists like Beth Stevens is focused on identifying new biomarkers that can signal the onset of Alzheimer’s earlier than current methods, potentially improving patient outcomes and transforming treatment strategies.
How does neuroscience contribute to our understanding of neurodegenerative diseases like Alzheimer’s?
Neuroscience breakthroughs in understanding the mechanisms of neurodegenerative diseases, including Alzheimer’s, are fundamental. Research like that conducted by Beth Stevens demonstrates how microglial functions and synaptic pruning are linked to disease progression, providing insights that could lead to innovative therapies.
What future research directions are suggested by Beth Stevens’ findings in Alzheimer’s disease?
Beth Stevens’ findings suggest that future research in Alzheimer’s disease should focus on the regulation of microglial activity and its impact on synaptic health. By developing targeted treatments that modulate microglial functions, researchers hope to slow or prevent the progression of Alzheimer’s and other neurodegenerative diseases.
| Key Point | Details |
|---|---|
| Research Focus | Understanding the role of microglial cells in the brain’s immune response, particularly how abnormal pruning affects neurodegenerative diseases. |
| Location | Boston Children’s Hospital and the Broad Institute of MIT and Harvard. |
| Key Findings | Identified the impact of excessive pruning on conditions like Alzheimer’s and Huntington’s disease, leading to potential new treatments and biomarkers. |
| Projected Impact | Research could influence treatment for approximately 7 million Americans living with Alzheimer’s disease. Potential rise in new cases by 2050 due to aging population. |
| Funding Support | Major support from federal agencies like the National Institutes of Health. |
| Long-Term View | Research often requires time to yield practical outcomes but is fundamentally essential for future advancements in treatment. |
| Quote from Beth Stevens | “I was just following the science… it’s this intriguing new field where the brain’s immune system was involved in sculpting synapses…” , |
Summary
Alzheimer’s disease research is at a crucial juncture, with transformative insights into microglial cells revealing their essential role in the brain’s immune defense. As demonstrated by Beth Stevens’ pioneering work, understanding how these cells interact with synapses is vital for developing new treatment strategies and identifying biomarkers for early detection. This fundamental research is instrumental in addressing the increasing challenges posed by Alzheimer’s, especially as the aging population contributes to a sharp rise in cases expected in the coming decades. The ongoing discoveries in this field highlight the importance of curiosity-driven science as a catalyst for significant medical advancements.